Product 1:
【1】Product Backgroud
| Product Name | 9-(3-Fluorophenyl)-5-(2-hydroxyethyl)-2,2-dimethyl-6,9-dihydro-[1,3]dioxolo[4,5-g]furo[3,4-b]quinolin-8(5H)-one |
| Appearance | white to light yellow solid |
| Solubility | Readily soluble in organic solvents (DMSO, DMF, dichloromethane), slightly soluble in water |
| Storage condition | Store in a dark, dry place at -20°C for a long time |
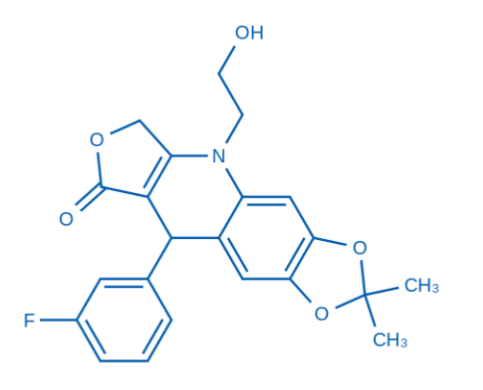
【2】Technical data
✓ It contains multifunctional groups such as fluorophenyl, hydroxyethyl, dioxacyclopentene and quinolinone, with a complex structure and may have significant biological activity
✓ Purity can reach more than 95%
![9-(3-Fluorophenyl)-5-(2-hydroxyethyl)-2,2-dimethyl-6,9-dihydro-[1,3]dioxolo[4,5-g]furo[3,4-b]quinolin-8(5H)-one.png](/../../upload/image/20251016/6389621018185034263590638.png)
【3】Technological advantage
✓ Suitable for mg to gram magnification
✓ The synthesis process is mature
【4】Commercial application
1. Medical field
Targeted drug development: Fluorine atoms and quinoline skeletons are commonly found in anti-cancer, anti-inflammatory or central nervous system drugs (such as kinase inhibitors or neuroprotectants).
Antibacterial/Antiviral: Compounds with similar structures may inhibit the enzyme activity of pathogens.
2. Materials Science
Fluorescent probes: Quinoline derivatives may be used in bioimaging or sensor materials.
3. Research purposes
It can be used as an intermediate to synthesize more complex molecules or for structure-activity relationship (SAR) research
Product 2
【1】Product Backgroud
| Product Name | N-[[P(S),2'R]-2'-Deoxy-2'-fluoro-2'-Methyl-6-O-Methyl-P-phenyl-5'-guanylyl]- L-alanine 1-Methylethyl ester structure |
| Cas no. | 1231747-08-2 |
| Molecular formula | C24H32FN6O8P |
| Molecular weight | 582.52 |
| Storage condition | Keep away from light at -20° C. For short-term use, store in a desiccator at 2-8°C |
| Stability | Sensitive to moisture, requires storage protected by dry inert gas |
| Appearance | White to off-white powder |
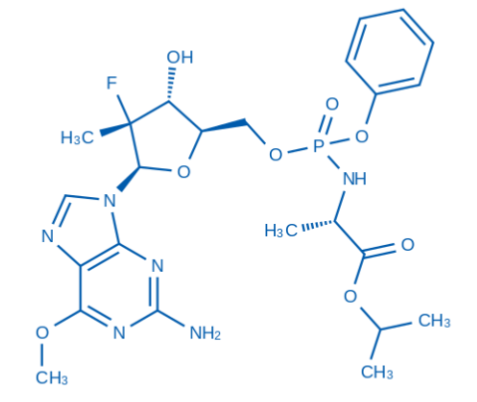
【2】Technical data
✓ Nucleotide modification: 2' -fluoro-2 '-methyldeoxyguanosine skeleton (similar to the structure of antiviral drugs, such as sofibuvir derivatives)
✓ Phosphate ester bond: Modified with p-phenyl and 6-O-methyl, it enhances lipid solubility and stability
✓ Amino acid conjugation: L-alanine isopropyl ester may improve cell permeability or prodrug properties
✓ Purification challenge: It is necessary to separate diastereomers (if present) by HPLC and confirm the structure by mass spectrometry /NMR
✓ Purity can reach more than 95%
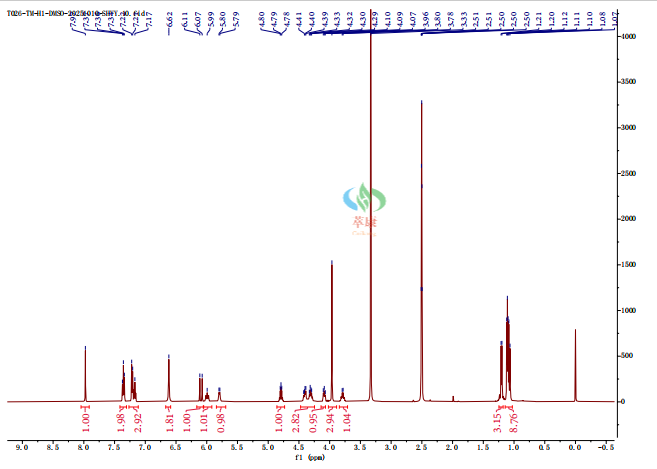
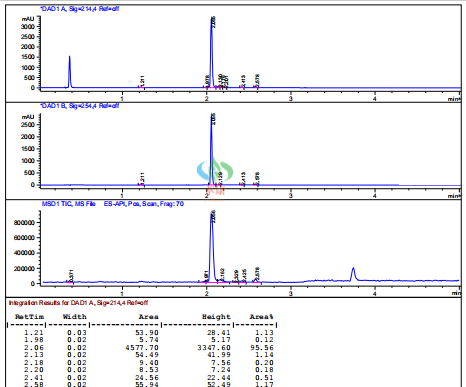
【3】Technological advantage
✓ Suitable for mg to gram magnification
✓ The synthesis process is mature
【4】Commercial application
1. Antiviral drug development
Target: RNA viral polymerases (such as HCV, influenza virus), 2' -modified nucleoside analogues can competitively inhibit viral replication
Prodrug design: The isopropyl ester protecting group may hydrolyze in the body to release the active component, enhancing oral bioavailability
2. Nucleic acid chemistry research
Modified nucleotide probes: used to study the stability of nucleases or epigenetic modification mechanisms
3. Drug delivery system
The structure of phenyl phosphate may be compatible with nanocarriers to achieve targeted delivery
Product 3
【1】Product Backgroud
| Product name | BB-Cl-Amidine |
| Cas no. | 1802637-39-3 |
| Molecular formula | C26H26ClN5O |
| Molecular weight | 459.971 |
| Appearance | white to beige |
| Chiral center | (S) -configuration, which may affect biological activity (enantiomer purity ≥98% is required) |
| Storage condition | Inert atmosphere,Store in freezer, under -20°C |
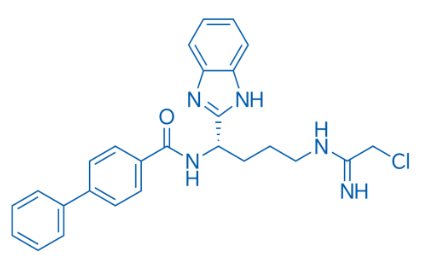
【2】Technical data
✓ Benzimidazole core: Commonly found in antiparasitic drugs (such as albendazole) or anti-cancer drugs.
✓ Chloroacetylimine: Potential alkylating agent, may participate in covalent bond binding targets.
✓ Biphenylamide: Enhances hydrophobicity and may regulate cell membrane permeability.
✓ Purity can reach more than 98%
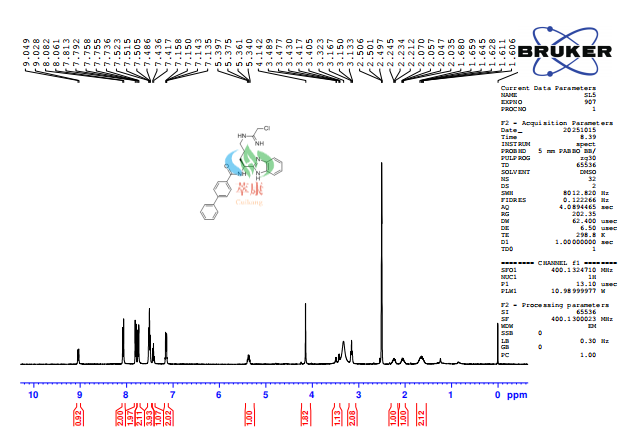
【3】Technological advantage
✓ Suitable for mg to gram magnification
✓ The synthesis process is mature
【4】Commercial application
1. Drug development
Anti-tumor candidate molecules: Similar structures can inhibit kinases (such as BTK) or topoisomerases.
Anti-infective agents: Benzimidazole derivatives are active against tubulin in fungi/parasites.
Covalent inhibitor precursors: Chloroacetyl groups may bind to the target protein cysteine through electrophilic attack.
2. Chemical biology tools
As a probe to study protein-small molecule interactions (fluorescence/biotin-labeled derivatization is required)
Product 4
【1】Product Backgroud
| Product Name | 1H-Imidazole-1-butanoic acid, α-amino-2-ethyl-α,4-dimethyl- |
| Cas no. | 2138524-92-0 |
| Molecular formula | C11H19N3O2 |
| Molecular weight | 225.29 |
| Boiling point | 437.719±45.00 °C |
| Storage condition | Dry and dark at 2-8°C, and fill with inert gas (such as argon) to extend stability |
| Appearance | Off-white solid |
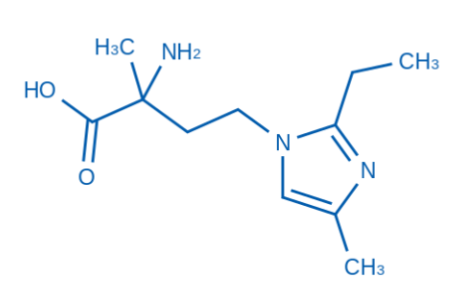
【2】Technical data
✓ Non-natural amino acid derivatives: α -carbon contains methyl side chains, which may affect bioavailability.
✓ Imidazole group: Endows amphoteric ionic properties (Ph-dependent solubility) and potential metal ion chelating ability.
✓ Chiral center: The 2-amino site needs to have a clear stereoconfiguration (such as R/S or racemic).
✓ Purity can reach more than 95%
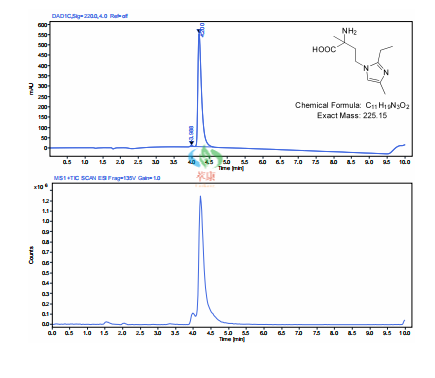
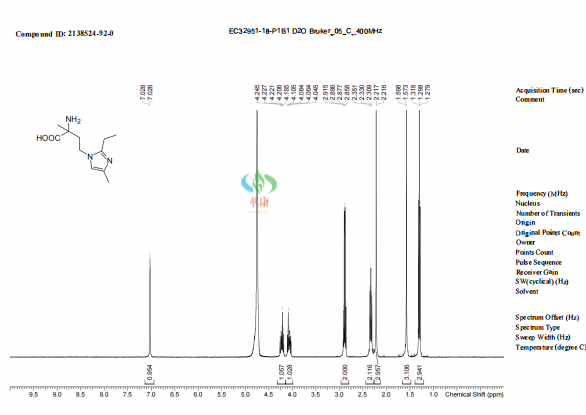
【3】Technological advantage
✓ Suitable for mg to gram magnification
✓ The synthesis process is mature
【4】Commercial application
1. Drug development
Targeted therapy: Similar structures are found in histamine receptor modulators or enzyme inhibitors (such as histidine decarboxylase inhibitors containing imidazoles)
Prodrug design: Carboxyl and amino groups can be derivatized into esters or amides to improve membrane permeability (such as the antifungal drug bifonazole analogues)
2. Chemical biology tools
Metalloproteinase probes: The imidazole group may simulate natural histidine residues and be used for the study of enzyme active sites
3. Materials Science
Coordination polymer construction: Porous materials are formed through the self-assembly of imidazole with metal ions (such as Zn²⁺, Cu²⁺)